Aptar’s Nasal Unidose System to Deliver neffy® (epinephrine nasal spray), the First and Only FDA-Approved Needle-Free Treatment for Type I Allergic Reactions, Including Anaphylaxis
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240815936474/en/
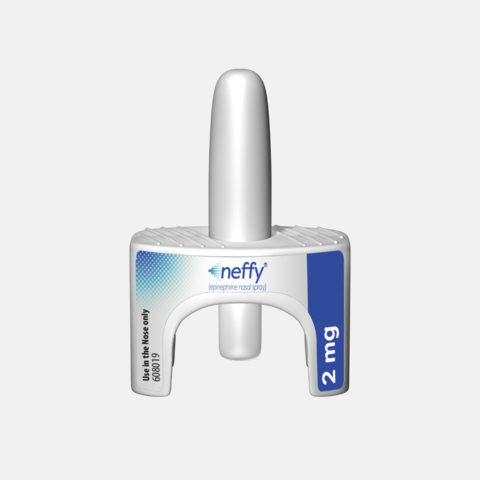
Photo: Aptar’s Unidose System for the neffy® 2 mg (epinephrine nasal spray). Image courtesy of ARS Pharmaceuticals.
Aptar’s Unidose is a single-use, ready-to-use, one-step nasal delivery system used to administer neffy to a patient during a severe allergic reaction. During such an event, the patient, healthcare professional, caregiver or user presses a small plunger on the bottom of the nasal spray system to release the drug in a single spray into the nostril.
Unidose (UDS) and Bidose (BDS) Technology Platforms
Aptar’s UDS and BDS technology platforms are designed to be robust, reliable and intuitive systems for easy administration by patients or caretakers. These drug delivery systems are designed and manufactured with strict quality controls intended to meet FDA’s guidelines for reliability. They offer biotech and pharmaceutical companies effective and reliable single or two-shot intranasal delivery for a variety of medicines including for emergency use and treatments of severe conditions. They can also be integrated with wireless connectivity technologies.
Accelerated Development Support via Aptar Pharma Services
This novel treatment for severe allergic reaction is an example of a Combination Product submission, and benefited from Aptar Pharma’s Services offering, a comprehensive portfolio of stage-specific development packages. Aptar’s dedicated Regulatory Affairs experts and analytical scientists help customers proactively address regulatory needs to help accelerate approval.
“The approval of neffy, which usesour Unidose System, and is the first nasally-delivered epinephrine treatment for severe allergic reaction, including anaphylaxis, once again demonstrates Aptar Pharma’s ‘formulation to patient’ focus on helping our customers develop complex, innovative treatments,” stated
About Aptar
Aptar Pharma is part of
About ARS Pharmaceuticals
ARS is a biopharmaceutical company dedicated to empowering at-risk patients and caregivers to better protect patients from severe allergic reactions that could lead to anaphylaxis. The company is commercializing neffy®, an epinephrine nasal spray product for patients with Type I allergic reactions, including food, medications and insect bites that could lead to life-threatening anaphylaxis. For more information, visit www.ars-pharma.com.
This press release contains forward-looking statements. Forward-looking statements generally can be identified by the fact that they do not relate strictly to historical or current facts and by use of words such as “expects,” “anticipates,” “believes,” “estimates,” “future,” “potential,” “continues” and other similar expressions or future or conditional verbs such as “will,” “should,” “would” and “could” are intended to identify such forward-looking statements. Forward-looking statements are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on our beliefs as well as assumptions made by and information currently available to us. Accordingly, our actual results or other events may differ materially from those expressed or implied in such forward-looking statements due to known or unknown risks and uncertainties that exist in our operations and business environment including, but not limited to: the successful integration of acquisitions; the regulatory environment; and competition, including technological advances. For additional information on these and other risks and uncertainties, please see our filings with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20240815936474/en/
Aptar Investor Relations Contact:
+1 347 351 6407
mary.skafidas@aptar.com
Aptar Pharma Media Contact:
+33 6 37 36 76 84
carolyn.penot@aptar.com
Aptar Media Contact:
+1 815 479 5671
katie.reardon@aptar.com
Source:
