WuXi Biologics Successfully Completes First Scale-Up of High-Productivity Bioprocessing Platform WuXiUI™ in 2,000L GMP Manufacturing
- By leveraging its ultra-intensified fed-batch platform, WuXiUI™,
WuXi Biologics has completed its first scale-up to 2,000L drug substance (DS) GMP manufacturing, achieving a 4-fold productivity improvement compared to the traditional fed-batch process - The competitive performance achieved through the utilization of both WuXiUI™ and the proprietary platform cell culture media MagniCHO™ led to significant reduction in overall DS manufacturing COGS
- The consistent performance of WuXiUI™ – from small scales to 2,000L GMP manufacturing – is a testament to the advancement of the technology as a mature and robust platform capable of significantly improving the cost-effectiveness of biologics production
In addition, the company's enhanced downstream technology platform enabled doubled purification processing capacity and similar impurity removal, which resulted in a 50% reduction in downstream processing time and a final DS yield of 70% with comparable product qualities. Moreover, WuXiUI™ downstream platform also enabled 30-50% reduction in the utilization of materials and consumables and therefore downsized waste generation. The improvement in total DS production contributed to significant reduction in manufacturing COGS, a substantial economic efficiency improvement in biologics production.
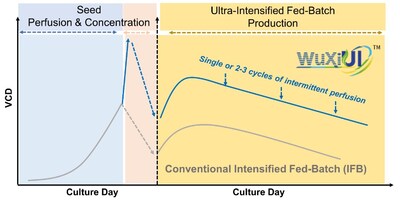
The WuXiUI™ platform, launched in 2023, enhances the productivity of multiple different CHO or other mammalian cell lines that express diverse product modalities, while maintaining desirable product qualities. It provides an effective solution for global clients to meet the growing demand for therapeutic proteins and antibodies with lower COGS. At the same time, it allows for a lower carbon footprint due to its more efficient media consumption, lower waste generation, and reduced demand for building space in the production line. The successful scale-up of the WuXiUI™ platform from bench scales to 2,000L GMP manufacturing confirms the robustness of the technology and its readiness for larger-scale production.
WuXiUI™'s strong performance was boosted through the application of MagniCHO™, WuXi Biologics' proprietary platform cell culture media enriched with nutrients for intensified processes. Furthermore, operation efficiency and production robustness were improved by integrating the Raman Process Analytical Technology (PAT) tool into the scale-up manufacturing, not only for process monitoring, but also – for the first time under GMP settings – to provide real-time automated process control.
Dr.
About
With over 12,000 skilled employees in
For more information about
Photo - https://mma.prnewswire.com/media/2493213/image.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/wuxi-biologics-successfully-completes-first-scale-up-of-high-productivity-bioprocessing-platform-wuxiui-in-2-000l-gmp-manufacturing-302234905.html
View original content:https://www.prnewswire.co.uk/news-releases/wuxi-biologics-successfully-completes-first-scale-up-of-high-productivity-bioprocessing-platform-wuxiui-in-2-000l-gmp-manufacturing-302234905.html

