FDA Issues Complete Response Letter for Biohaven's VYGLXIA (troriluzole) New Drug Application for Spinocerebellar Ataxia
-
The troriluzole clinical development program encompassed the first industry clinical trials to generate data showing therapeutic potential in patients with spinocerebellar ataxia (SCA), a rare genetic, inherited, life-threatening neurodegenerative disease with no treatment options.
-
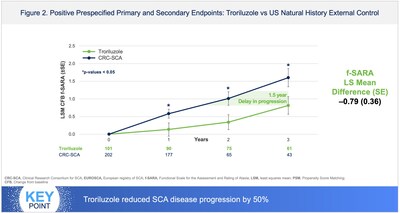
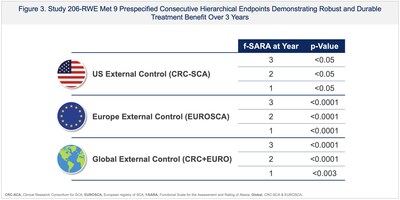
Compelling data from troriluzole's new drug application (NDA) included: a 3-year real-world evidence study (Study 206-RWE) showing slowing of SCA disease progression by 50-70% in troriluzole-treated patients compared to matched untreated external controls; > 50% risk reduction in AEs of falls in troriluzole-treated subjects compared to placebo from the safety analysis of the 1-year, double-blind, placebo controlled Study -206; and multiple supportive analyses showing a delay in becoming wheelchair bound or losing the ability to walk, decreased gait impairment as measured by f-SARA and objective video-based kinematic analysis, and improvement in overall functioning as assessed by the clinician global impression (CGI) scale.
-
The FDA issued a complete response letter (CRL) despite Study 206-RWE being reviewed by FDA and achieving statistical significance in the study's prespecified primary and secondary outcome efficacy endpoints. FDA cited issues that can be inherent to real-world evidence and external control studies including potential bias, design flaws, lack of pre-specification and unmeasured confounding factors.
-
Prior to 206-RWE protocol finalization, study approval and topline data analysis, the FDA provided feedback on the study's statistical analysis plan and study protocol that were incorporated into the IRB approved study. The FDA official meeting minutes (
March 8, 2024 ) from discussion of the RWE study included the statement, "a large and robust treatment effect would be needed to overcome the biases of an externally controlled trial, in order for it to be used as the primary basis for substantial evidence for effectiveness." -
Biohaven the United States andEurope , clearly met criteria of "a large and robust treatment effect."
-
Prior to 206-RWE protocol finalization, study approval and topline data analysis, the FDA provided feedback on the study's statistical analysis plan and study protocol that were incorporated into the IRB approved study. The FDA official meeting minutes (
-
Troriluzole received Orphan and Fast Track designation as well as a Priority Review acceptance of the NDA; FDA subsequently delayed the PDUFA date by 3 months during the review period. There was no communication of the need for an Advisory Committee meeting at acceptance of the Priority Review; however, a few months later the FDA informed
Biohaven that an Advisory Committee was being planned but then cancelled it weeks before the anticipated meeting, preventing qualified clinical experts the opportunity to publicly weigh in on their opinion of what is a large and robust treatment effect and after the Company spent significant resources preparing for the Advisory Committee.
-
Compelling data from troriluzole's new drug application (NDA) included: a 3-year real-world evidence study (Study 206-RWE) showing slowing of SCA disease progression by 50-70% in troriluzole-treated patients compared to matched untreated external controls; > 50% risk reduction in AEs of falls in troriluzole-treated subjects compared to placebo from the safety analysis of the 1-year, double-blind, placebo controlled Study -206; and multiple supportive analyses showing a delay in becoming wheelchair bound or losing the ability to walk, decreased gait impairment as measured by f-SARA and objective video-based kinematic analysis, and improvement in overall functioning as assessed by the clinician global impression (CGI) scale.
-
In the CRL, the FDA recommended that Biohaven meet with the Division to discuss the evidence that will be needed to support a future NDA for the treatment of SCA with troriluzole. Following receipt of the CRL today,
Biohaven is in the process of formally requesting a meeting as soon as possible given the large number of patients who are currently being treated in the expanded access program. -
Biohaven remains committed to working with the FDA to find a path forward for its NDA for VYGLXIA and plans to meet with the FDA to discuss potential next steps. -
Given the CRL,
Biohaven is initiating strategic portfolio and cost-optimization measures to prioritize 3 key, late-stage, clinical programs with the greatest potential for value generation:
- Key areas of focus over the near term include: 1) Clinical-stage, lead extracellular degraders for IgA nephropathy (BHV-1400) and Graves' disease (BHV-1300); 2) Opakalim, Kv7 ion channel activator, pivotal trials in adult focal epilepsy and depression; and 3) Taldefgrobep alfa, myostatin-activin pathway inhibitor for obesity and SMA.
- Restructuring of business priorities underway to achieve an approximately 60% reduction in annual direct R&D spend (which excludes personnel and SBC), will result in delay of non-priority programs.
-
New data will be presented from several of
Biohaven's priority programs at an annual healthcare conference inJanuary 2026 .
Prioritizing Clinical-Stage, Innovative Assets
Key areas of focus over the next year will include:
1) Clinical-stage, lead extracellular degraders for IgA nephropathy (BHV-1400) and Graves' disease (BHV-1300);
2) Opakalim, Kv7 ion channel activator, pivotal trials in adult focal epilepsy and depression; and
3) Taldefgrobep alfa, myostatin-activin pathway inhibitor, for SMA and obesity.
About Spinocerebellar Ataxia (SCA)
Spinocerebellar ataxia is a group of dominantly inherited neurodegenerative disorders characterized by progressive loss of voluntary motor control and atrophy of the cerebellum and brainstem. SCA affects approximately 15,000 people in the United States and 24,000 in Europe and the United Kingdom. Patients experience significant morbidity, including impaired gait leading to falls, loss of ambulation and progression to a wheelchair, inability to communicate due to speech impairment, difficulty swallowing, and premature death. While signs and symptoms can appear anytime from childhood to late adulthood, SCA typically presents in early adulthood and progresses over a number of years. Currently, there are no FDA-approved treatments and no cure for SCA.
About Troriluzole
Troriluzole is a new chemical entity (NCE) and third-generation novel prodrug that modulates glutamate, the most abundant excitatory neurotransmitter in the human body. The primary mode of action of troriluzole is reducing synaptic levels of glutamate. Troriluzole increases glutamate uptake from the synapse, by augmenting the expression and function of excitatory amino acid transporters located on glial cells that play a key role in clearing glutamate from the synapse. The glutamate modulating activity of troriluzole addresses the widely documented glutamate deregulation that underlies neurodegeneration and Purkinje cell dysfunction in patients with SCA. Troriluzole also has the potential to be developed in a number of other diseases associated with excessive glutamate. More information about troriluzole can be found at the Biohaven's website: https://www.biohaven.com/pipeline/glutamate/
About Biohaven
Biohaven is a biopharmaceutical company focused on the discovery, development and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience and oncology. Biohaven is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. Biohaven's key clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; MoDE™ and TRAP™ extracellular protein degradation for immunological diseases; and myostatin-activin pathway targeting agent for neuromuscular and metabolic diseases, including SMA and obesity. For more information, visit www.biohaven.com.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding the expected timing and amounts of funding under the NPA. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate", "potential first-in-class" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates and regarding reduction in annual direct R&D spend, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of
VYGLXIA is a registered trademark, and MoDE and TRAP are trademarks, of
Investor Contact:
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/fda-issues-complete-response-letter-for-biohavens-vyglxia-troriluzole-new-drug-application-for-spinocerebellar-ataxia-302604884.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/fda-issues-complete-response-letter-for-biohavens-vyglxia-troriluzole-new-drug-application-for-spinocerebellar-ataxia-302604884.html
SOURCE