Biohaven Highlights Portfolio Progress, Positive Early Patient Data from Priority Degrader Programs and Anticipated Milestones at the 44th Annual J.P. Morgan Healthcare Conference
-
Reports positive early clinical experience from first and only clinically validated, extracellular protein degraders using
Biohaven 'sYale University . Early clinical experience in patients demonstrates rapid removal of circulating disease-causing proteins, resulting in robust clinical improvement with a well-tolerated profile to date:-
IgA Nephropathy (IgAN) Program: First dosing of BHV-1400 TRAP degrader in IgAN patients achieved early observations of both biomarker and clinical responses including: selective lowering of only the disease-causing galactose-deficient IgA1 (Gd-IgA1) while sparing off-target effects on healthy antibodies (IgA, IgM, IgE, IgG), resolution of blood in the urine (hematuria), deep reductions in proteinuria, and improvement in fatigue and kidney function (eGFR) within weeks.
- Completed 4Q25 FDA meeting to align on pivotal IgAN study design; study to initiate in early 2026
-
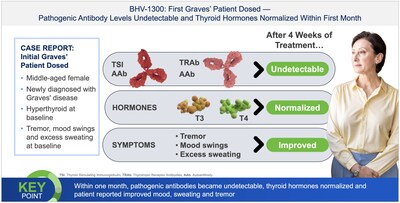
Graves' Disease Program: First-in-patient clinical experience with BHV-1300, an IgG MoDE degrader, resulted in a complete suppression of disease-causing TSH receptor-stimulating antibodies with accompanying normalization of previously elevated thyroid hormones within weeks after dosing a patient with Graves' disease. BHV-1300 has shown the potential for best-in-class reductions of IgG, with maximum reductions of up to an 87% decrease from baseline within weeks of dosing.
Biohaven is planning a pivotal study of BHV-1300 in Graves' disease in 2026.
-
Advancement of Broad Degrader Portfolio: With clinical proof-of-concept now established,
Biohaven is positioned to advance multiple next-generation degraders targeting high value immune-mediated disease indications and explore strategic partnerships to advance the breadth of this platform technology.
-
IgA Nephropathy (IgAN) Program: First dosing of BHV-1400 TRAP degrader in IgAN patients achieved early observations of both biomarker and clinical responses including: selective lowering of only the disease-causing galactose-deficient IgA1 (Gd-IgA1) while sparing off-target effects on healthy antibodies (IgA, IgM, IgE, IgG), resolution of blood in the urine (hematuria), deep reductions in proteinuria, and improvement in fatigue and kidney function (eGFR) within weeks.
-
Announces preliminary data with opakalim, a selective Kv7 channel activator, in an ongoing open-label extension (OLE) clinical study in focal epilepsy.
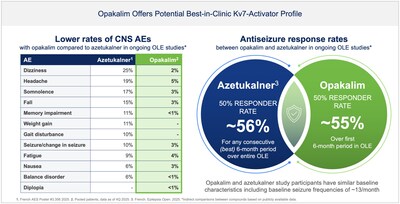
- Demonstrated clinically meaningful reductions in seizure frequency compared to pretreatment baseline. Specifically, the majority of participants treated with opakalim 75 mg once daily who completed at least 6 months of OLE treatment showed ≥50% reductions in seizure frequency compared to pretreatment baseline.
- Well-tolerated in the OLE with a low incidence of central nervous system adverse events, representing a potential paradigm-shift for patients with a highly favorable and differentiated safety profile compared to other approved or investigational antiseizure medicines.
- Pivotal results for opakalim in the treatment of focal epilepsy expected in 2026.
-
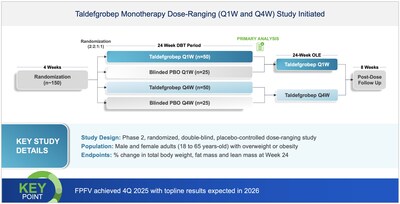
Announces initiation of obesity Phase 2 study with
Biohaven's myostatin-activin inhibitor targeting high quality weight loss. Dosing began in 4Q 2025 with topline results expected in 2026- Taldefgrobep alfa, an inhibitor of the myostatin-activin II receptor signaling pathways, directly targets: fat reduction, increased lean muscle mass and improves in bone density while avoiding intolerable adverse effects.
- Taldefgrobep-myostatin complexes also result in the inhibition of Activin E - ALK7 in adipocytes, thereby having a direct effect on reducing adipose tissue.
- Taldefgrobep has demonstrated a highly favorable safety and tolerability profile in >700 clinical trial participants studied to date.
-
Highlights clinical progress with
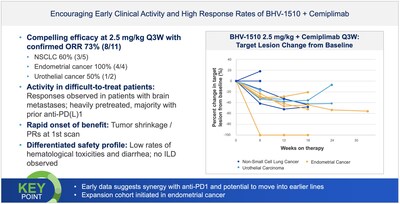
Biohaven's next generation antibody drug conjugates (ADCs) in oncology- Phase 1/2 study evaluating BHV-1510 (Trop2 ADC) as monotherapy and in combination with Regeneron's anti-PD-1 monoclonal antibody cemiplimab shows continued clinical activity with confirmed responses and a differentiated safety profile in the ongoing study.
- Endometrial cancer expansion cohort has been initiated with the combination of BHV-1510 and cemiplimab.
- Initiated novel subcutaneous dosing with BHV-1510, first and only Trop2 ADC in clinic with potential subcutaneous route of administration.
- First-in-class FGFR3 directed ADC, BHV-1530, continues with dose escalation and no dose limiting toxicities to date.
-
Advancements across portfolio of early clinical and next-generation future programs, including:
- Pivotal clinical trial continues to advance enrollment with
Biohaven's brain-penetrant TYK2/JAK1 inhibitor, BHV-8000 to treat early Parkinson's disease. - BHV-1955, a novel, long-acting intranasal formulation of oxytocin, for the central treatment of tinnitus.
- BHV-8100, a brain-penetrant activator of the M2 isoform of pyruvate kinase (PKM2) for neurodegenerative disorders and aging.
- Pivotal clinical trial continues to advance enrollment with
"We've dosed our first patient in our obesity program with taldefgrobep that introduces a dual-action therapy to not only reduce fat but also build lean muscle mass, addressing a critical unmet need in metabolic health," continued Dr. Coric "We expect topline data from this Phase 2 obesity proof-of-concept trial in 2026. In addition, we continue to advance a clinically validated approach for treating focal epilepsy that targets Kv7 ion channels with pivotal topline data expected in 2026. Our advancements and discovery expertise demonstrate our commitment to developing innovative treatments that address a wide range of patient needs."
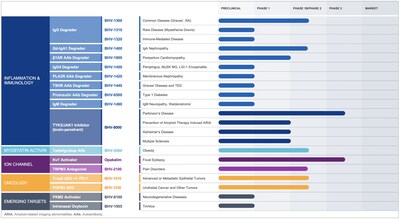
Novel Degrader Platforms: Biohaven Raises the Bar in Precision Immunology
As part of an initiative to further advance and accelerate next-generation degrader development,
BHV-1400: Next-Generation TRAP Degrader for IgA Nephropathy (IgAN)
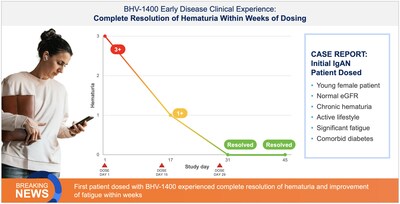
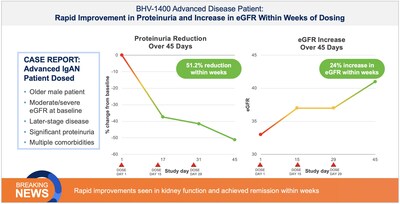
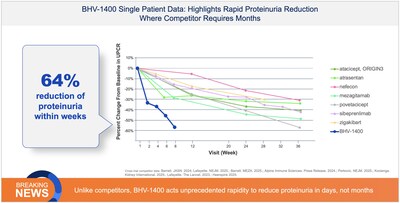
The first clinical experience with BHV-1400 underscores its paradigm-shifting potential to selectively remove only aberrant Gd-IgA1, the disease-causing species in IgA nephropathy, while sparing normal IgA. First-in-patient dosing with BHV-1400, a next-generation TRAP degrader, achieved selective lowering of Gd-1gA1 within hours, translating to hematuria resolution, deep reductions in proteinuria, and eGFR improvements within weeks of dosing.
The first patient dosed, a young woman with early disease, normal baseline eGFR and chronic hematuria experienced rapid reductions in Gd-IgA1 associated with complete resolution of chronic hematuria and improvement in symptoms of fatigue within weeks of dosing. The second patient dosed, a man with late-moderate-to-severe loss of kidney function as evidenced by decreased baseline eGFR, experienced rapid reductions in Gd-IgA1 associated with robust reductions in proteinuria of greater than 60% and improvement in kidney function as measured by a 24% increase in GFR within weeks of dosing. Importantly, both patients showed no significant reductions in normal IgA, IgG, or IgM. Additional IgAN patients are being treated in the expansion cohort and plans are underway for initiating a pivotal trial of BHV-1400 in 2026.
In the ongoing Phase 1, BHV-1400 achieved rapid lowering of Gd-IgA1 with a mean reduction of >60% within hours. Maximum reduction exceeding 80% was observed following the first dose. This selective and rapid approach to immunoglobulin lowering represents a second-generation therapeutic approach to IgAN, potentially allowing for faster, meaningful disease control with less acute or long-term safety risks associated with complement inhibition or broad antibody suppression. As in healthy participants, BHV-1400 has been safe and well-tolerated in initial patients with IgAN. As of
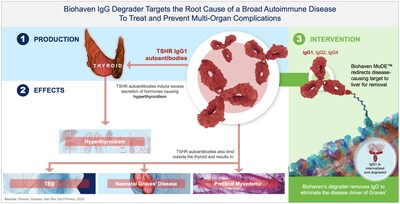
BHV 1300: MoDE Degrader with Potential to Transform Care Across Multiple IgG Mediated Diseases
BHV-1300,
Results from the first-in patient clinical experience with BHV-1300 also showed that it induced complete suppression of pathogenic TSH receptor-stimulating antibodies with normalization of previously elevated thyroid hormones within weeks after dosing. There were no clinically significant reductions in IgG3, IgA, IgE, IgM, or albumin, nor increases in cholesterol compared to baseline. Clinical findings to date position BHV-1300 for pivotal study initiation in Graves' disease in 2026, as well as additional follow-on studies in other autoimmune diseases where its therapeutic pan-IgG depletion capability is expected to have significant potential.
Biohaven Discovery Engine for Next-Generation Degraders
With proof-of-concept now established in patients for the first clinically validated extracellular protein degrader platform,
- BHV-1420, a PLA2R autoantibody specific TRAP degrader, for membranous nephropathy;
- BHV-1450, a IgG4 specific MODE degrader, for potential indications including pemphigus vulgaris and myasthenia gravis with anti-MuSK antibodies;
- BHV-1440, a TSHR autoantibody specific TRAP degrader, as the next-generation of immune therapy for Graves' disease and thyroid eye disease;
- BHV-6500, a proinsulin and insulin autoantibody TRAP degrader, for type 1 diabetes;
- BHV-1490, an IgM MoDE degrader for cryoglobulinemia, Waldenstrom's macroglobulinemia and IgM neuropathy;
- BHV-1310, a next generation IgG MoDE degrader, for management of rare IgG-mediated indications;
- BHV-1600, a beta-1 adrenergic receptor autoantibody degrader, for cardiomyopathy; and,
- Multiple undisclosed degrader targets in early discovery development.
Epilepsy Program: Revolutionizing the Treatment of Epilepsy with a Modern Kv7 Activator
Opakalim is a next-generation, selective Kv7 activator, targeting a clinically validated mechanism of action for the treatment of epilepsy. It is highly differentiated from ezogabine, a first-generation non-selective Kv7 activator previously approved for the treatment of focal seizures, and ezogabine analogs in development. Importantly, opakalim does not exhibit the GABA receptor activity seen with ezogabine and other antiseizure medicines (ASMs), which contribute to central nervous system (CNS) adverse effects and their poor tolerability in the clinic. Opakalim is being developed to address the significant unmet medical need that exists for novel ASMs having a favorable tolerability profile, especially those with mechanisms of action that are complementary to currently available ASMs.
Review of data from the ongoing open-label clinical trial experience with opakalim in focal epilepsy support the potential for opakalim to achieve compelling efficacy and to deliver a highly favorable and differentiated safety profile. Open-label treatment with opakalim demonstrated clinically meaningful reductions in seizure frequency compared to the pretreatment baseline observation period prior to randomization. Specifically, 55% of participants showed ≥50% reductions in seizure frequency (≥50% responder rate), for those who completed at least 6 months of treatment with opakalim 75 mg once daily in the open-label study; and this result is comparable to the ≥50% responder rate published for other investigational agents in the class such azetukalner (which has reported 56% of patients with a ≥50% responder rate over any consecutive best 6-month period from its Phase 2b OLE data). Notably, the antiseizure effects of opakalim were correlated with plasma concentrations, based on a preliminary exposure-response analysis. Opakalim was well-tolerated in the open-label study with a low incidence of CNS adverse events, consistent with prior studies with opakalim.
These preliminary observations support the paradigm-shifting potential of opakalim to achieve antiseizure efficacy with a highly favorable and differentiated tolerability profile compared to other approved ASMs and those in development.
- Pivotal topline results for opakalim in the treatment of focal epilepsy are expected in 2026.
Obesity Program: Targeting High Quality Weight Loss
Taldefgrobep alfa is
Taldefgrobep's differentiated mode of action targets fat reduction, builds muscle, and increases bone density.
In the clinic, taldefgrobep has demonstrated beneficial changes in fat mass and lean mass in non-obese populations, including healthy adult participants in a Phase 1 study. Participants who received taldefgrobep once-weekly realized significant reductions in total body fat mass (>6%) and increases in lean muscle mass (up to 4%) after one month of dosing. Notably, these body composition parameters continued to demonstrate additional improvements after cessation of dosing associated with the persistence of the pharmacologically active taldefgrobep-myostatin complex and suggesting the drug may support extended dosing intervals. Recent nonclinical data demonstrates that this complex cam also potently inhibit the Activin E-ALK7 signaling axis within adipocytes, further underpinning the complementary mechanistic advantages of taldefgrobep in both growing muscle and reducing fat.
Taldefgrobep has an established safety profile that is well-suited for an indication in overweight and obesity. It has been previously evaluated in >700 clinical trial participants across 5 completed studies and an ongoing Phase 3 trial in spinal muscular atrophy. Throughout its clinical development, taldefgrobep has been well tolerated, with rates of muscle and gastrointestinal adverse events comparable to placebo.
- Phase 2 topline results for taldefgrobep in the treatment of obesity are expected in 2026.
Advancement of Key Next Generation ADC Oncology Programs
BHV-1510 (Trop2 ADC): Preliminary Phase 1 results for BHV-1510 showed encouraging clinical activity with confirmed responses and a differentiated safety profile demonstrating clinical proof of principle with
BHV-1530 (FGFR3 ADC):
Progress across portfolio of clinical and next-generation discovery programs:
Patient enrollment continues in pivotal Phase 2/3 clinical trial in early Parkinson's disease with brain-penetrant TYK2/JAK1 inhibitor, BHV 8000.
Biohaven Discovery Engine: Next wave of innovative, therapeutics primed to advance forward at an intentional cadence.
- Discovery has advanced multiple novel development candidates for future clinical development. Beyond the degrader platform, these include:
- BHV-1955 targeting the oxytocin receptor centrally for the treatment of tinnitus;
- BHV-2120, oral small molecule TRPM3 inhibitor for epilepsy
- BHV-8555, a brain penetrant, oral small molecule preventing a-synuclein aggregation in Parkinson's disease; and
- BHV-8100, a brain-penetrant, oral small molecule activating the M2 isoform of pyruvate kinase (PKM2) for neurodegenerative disorders and aging.
About
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of
MoDE and TRAP are trademarks of Biohaven Therapeutics Ltd.
Investor Contact:
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Sam
christycurran@sambrown.com
615-414-8668
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-highlights-portfolio-progress-positive-early-patient-data-from-priority-degrader-programs-and-anticipated-milestones-at-the-44th-annual-jp-morgan-healthcare-conference-302658655.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-highlights-portfolio-progress-positive-early-patient-data-from-priority-degrader-programs-and-anticipated-milestones-at-the-44th-annual-jp-morgan-healthcare-conference-302658655.html
SOURCE